-
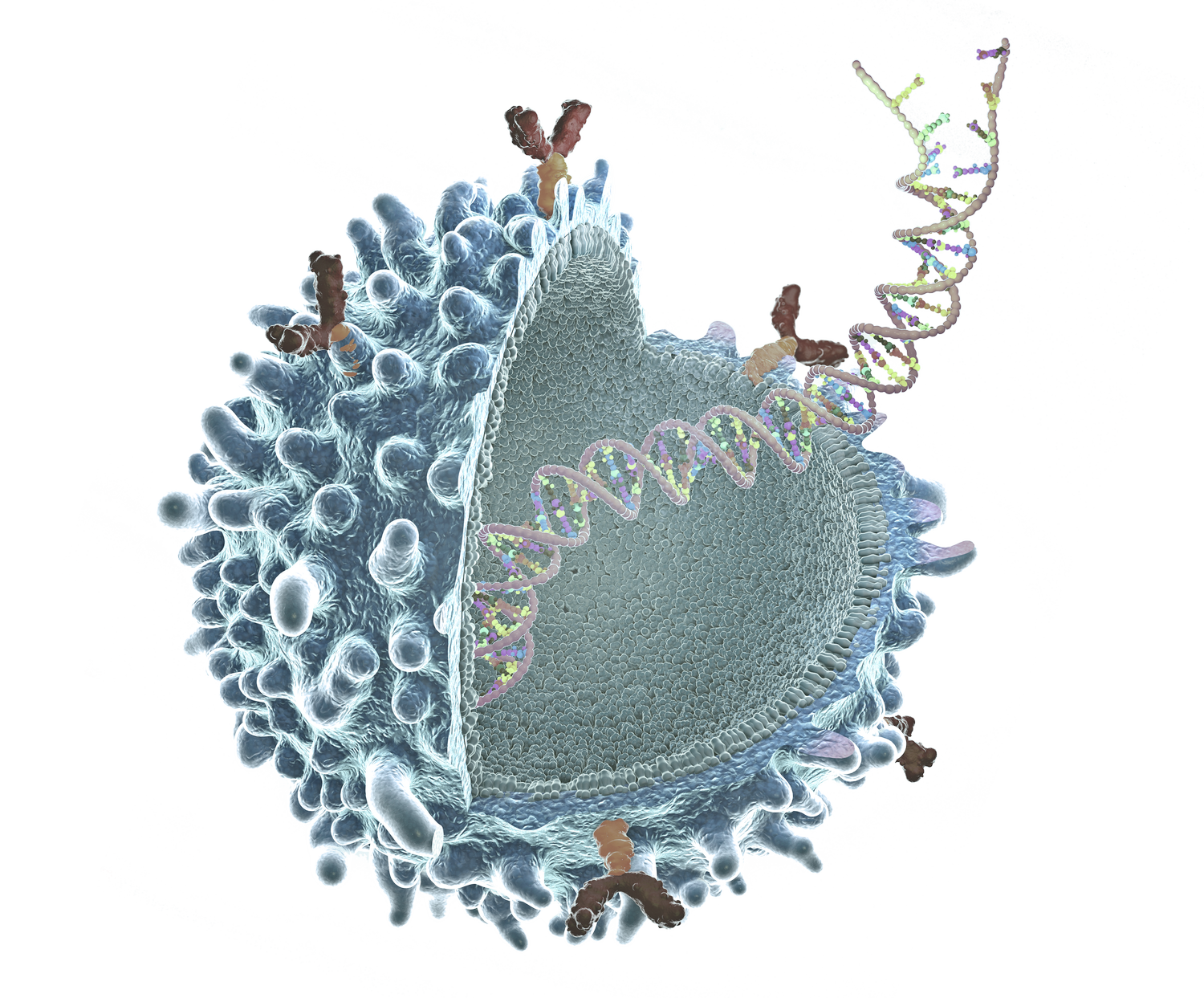
Rapid detection and quantification of cellular properties during viral vector production either via plasmid transfection (AAV, lentivirus) or viral infection (baculovirus, adenovirus, or HSV), including titer and packaging efficiency. In process results provided by Radiance® can be correlated to existing assays such as ddPCR, ELISA, or TCID50 to provide results in just minutes. This improves the speed and quality of process development by reducing time to results and the need to run tedious and labor-intensive assays that require significant time and resources.
-
When used as a process analytical technology (PAT) during production/manufacturing, Radiance® can provide rapid information on how input cell and virus raw materials vary over time, and then real-time analytics to help scientists understand performance and variation throughout a production run, thereby increasing process knowledge to ensure consistent performance and reduce the occurrence of out of spec batches. Radiance® is GMP ready and designed for ease of use by production personnel. Both the Illuminate software that powers Radiance® and LumaCyte’s cloud-based analytics platform ReportR are 21 CFR Part 11 compliant.
-
For analytical development, Radiance® provides viral transduction, infectivity and other cell-based assays for gene therapy R&D, process development and optimization, formulations, and potency. Precise viral analytics allow for more accurate vial filling to prevent the need for over formulation and provide a better prediction of shelf life.
-
Radiance® enables label-free viral neutralization assays with high accuracy and precision as well as a large dynamic range for clinical trial and other applications. This facilitates the rapid screening of patients for previous exposure to viral vectors as well as the evaluation of immunological response as a result of treatment.